Highlighted Publications
For a complete list of publications, please take a look at the links below.
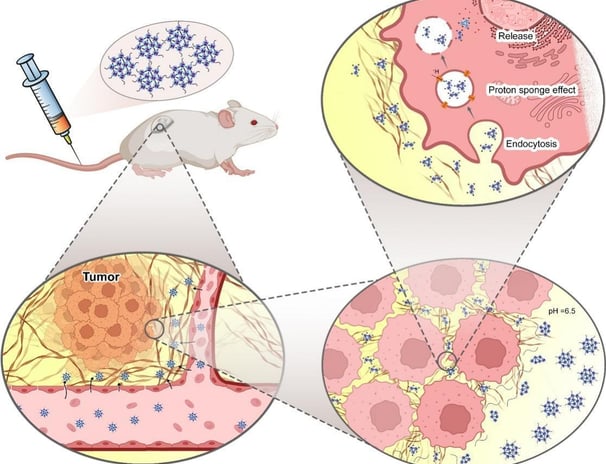
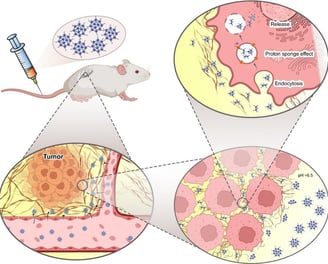
Switchable PAMAM megamers for deep tumor penetration and enhanced cell uptake
Nanoparticles fabricated to deliver anticancer drugs are usually designed to present optimized tumor penetration and cell internalization. However, there are some barriers and difficulties with most current technologies. Herein, size and charge switchable polyamidoamine (PAMAM) megamers (SChPMs) were prepared for the delivery of doxorubicin (DOX). SChPMs were fabricated by connecting PAMAM dendrimers with pH-sensitive bonds and surface PEGylation. At pH 7.4, the size and surface charge of these nanocarriers were approximately 100 nm and + 0.75 mV, but at the acidic extracellular pH of tumor cells (pH 6.5), their size were reduced dramatically (15 nm) and their surface charge increased to +6.7 mV. Cell studies confirmed that alteration of the size and surface charge enhanced their penetration into multicellular spheroids and cell internalization. These megamers, in addition to delivering the drug to the deeper areas of the tumor, could powerfully overcome physiological resistance to anthracycline-based drugs. The nanocarrier revealed enhanced antitumoral activity in animal studies. Toxicology studies and histopathological assessments of vital tissues of 4 T1 tumor bearing mice indicated minimal tissue damage when DOX-loaded SChPMs (DSChPMs) were used. It can be concluded that the versatile and agile nanocarriers developed in this study could be considered for further investigations into their clinical application.
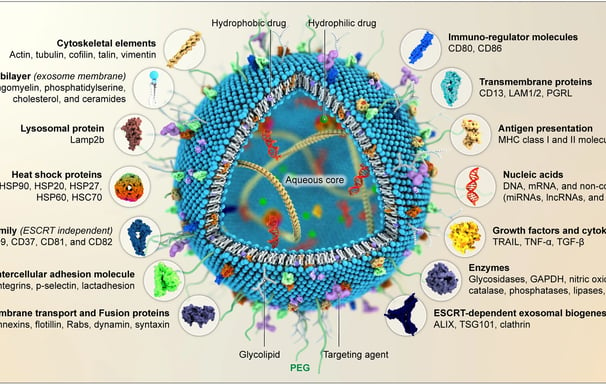
Exosomes as therapeutic and drug delivery vehicle for neurodegenerative diseases
Neurodegenerative disorders are complex, progressive, and life-threatening. They cause mortality and disability for millions of people worldwide. Appropriate treatment for neurodegenerative diseases (NDs) is still clinically lacking due to the presence of the blood-brain barrier (BBB). Developing an effective transport system that can cross the BBB and enhance the therapeutic effect of neuroprotective agents has been a major challenge for NDs. Exosomes are endogenous nano-sized vesicles that naturally carry biomolecular cargoes. Many studies have indicated that exosome content, particularly microRNAs (miRNAs), possess biological activities by targeting several signaling pathways involved in apoptosis, inflammation, autophagy, and oxidative stress. Exosome content can influence cellular function in healthy or pathological ways. Furthermore, since exosomes reflect the features of the parental cells, their cargoes offer opportunities for early diagnosis and therapeutic intervention of diseases. Exosomes have unique characteristics that make them ideal for delivering drugs directly to the brain. These characteristics include the ability to pass through the BBB, biocompatibility, stability, and innate targeting properties. This review emphasizes the role of exosomes in alleviating NDs and discusses the associated signaling pathways and molecular mechanisms. Furthermore, the unique biological features of exosomes, making them a promising natural transporter for delivering various medications to the brain to combat several NDs, are also discussed.
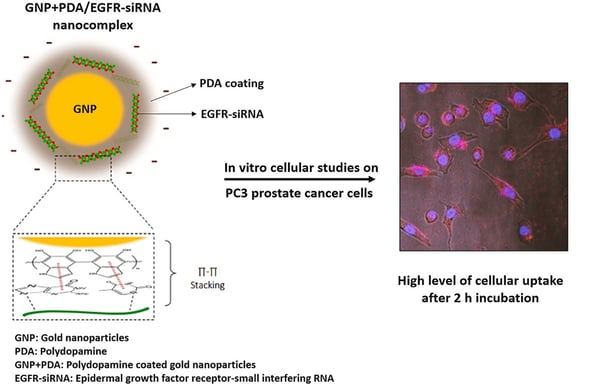
Delivery of EGFR-siRNA to prostatic cancerous cells based on polydopamine coated gold nanoparticles
Common treatment of metastatic prostate cancer is limited to androgen deprivation (AD) therapy and chemotherapeutic agents, while the 5-year survival rate is less than 30%. The role of EGFR in metastasis and drug resistance of prostate cancer has been well reported. In this regard, small interfering RNA (siRNA) could be an efficacious strategy for improving the therapeutic outcomes against metastatic prostate cancer. However, the clinical application of siRNA over the decades has not been achieved due to various challenges such as stability, targeting, and transfection into the cancer cells. To overcome these, herein a nanocarrier delivery system for EGFR-siRNA based on gold nanoparticles coated with polydopamine (GNP-PDA) has been developed. The nanocarrier was prepared by polymerization of dopamine, a mussel adhesive protein, on the surface of GNPs. The GNP-PDA was able to properly complex with EGFR-siRNA. The GNP-PDA/EGFR-siRNA, with 55.59 ± 11.53 nm particle size and a negative surface charge, could retard the siRNA for 2 h studied through gel retardation assay. The flowcytometry and confocal microscopy demonstrated a high cellular uptake (>93%) over 2 h incubation with PC3 cells. The MTT assay further revealed that the PDA coating decreases the cytotoxicity of GNPs significantly. The GNP-PDA/EGFR-siRNA nanocomplex, at low concentration, showed 60% cytotoxicity, 55% apoptosis and 50.9% gene knockdown after 24 h incubation of the samples in PC3 cells. In conclusion, polydopamine-coated gold nanoparticles could be applied as a safe and efficient nano carrier for siRNA delivery to solid tumors.
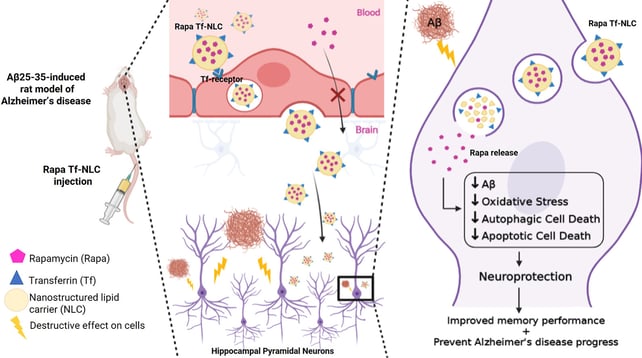
Transferrin decorated-nanostructured lipid carriers (NLCs) are a promising delivery system for rapamycin in Alzheimer's disease: An in vivo study
Alzheimer's disease (AD), the most common neurodegenerative disorder, is characterized by progressive cognitive impairment and memory loss. The mammalian target of rapamycin (mTOR) signaling pathway could regulate learning and memory. The effect of rapamycin (Rapa) on mTOR activity could slow or prevent the progression of AD by affecting various essential cellular processes. Previously, we prepared transferrin (Tf) decorated-nanostructured lipid carriers (NLCs) for rapamycin (150 ± 9 nm) to protect the drug from chemical and enzymatic degradation and for brain targeted delivery of rapamycin. Herein, the effect of Tf-NLCs compared to untargeted anionic-NLCs and free rapamycin, were studied in amyloid beta (Aβ) induced rat model of AD. Behavioral test revealed that the Rapa Tf-NLCs were able to significantly improve the impaired spatial memory induced by Aβ. Histopathological studies of hippocampus also showed neural survival in Rapa Tf-NLCs treated group. The immunosuppressive, and delayed wound healing adverse effects in the rapamycin solution treated group were abolished by incorporating the drug into NLCs. The Aβ induced oxidative stress was also reduced by Rapa Tf-NLCs. Molecular studies on the level of Aβ, autophagy (LC3) and apoptotic (caspase-3) markers, and mTOR activity revealed that the Rapa Tf-NLCs decreased the Aβ level and suppressed the toxic effects of Aβ plaques by modulating the mTOR activity and autophagy, and decreasing the apoptosis level. As a conclusion, the designed Tf-NLCs could be an appropriate and a safe brain delivery system for rapamycin and make this drug more efficient in AD for improving memory and neuroprotection.
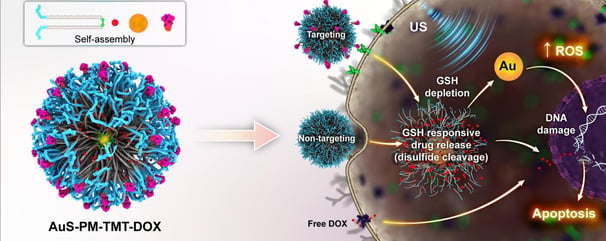
Targeted pH- and redox-responsive AuS/micelles with low CMC for highly efficient sonodynamic therapy of metastatic breast cancer
The efficacy of injectable micellar carriers is hindered due to the disassembly of micelles into free surfactants in the body, resulting in their dilution below the critical micelle concentration (CMC). Copolymer micelles were developed to address this issue, containing a superhydrophilic zwitterionic block and a superhydrophobic block with a disulfide bond, which exhibited a CMC lower than conventional micellar carriers. Cleavable copolymers composed of 2-methacryloyloxyethyl phosphorylcholine (MPC) zwitterion and polycaprolactone CHLZW as the shell, with gold nanoparticles as their core, were studied to deliver doxorubicin to tumor cells while reducing the side effect of the free cytotoxic agent. The research focused on the impact of gold nanoparticles present in targeted TMT-micelles core on stability and in vivo bioavailability and sonotoxicity of the nanoparticles, as well as their synergistic effect on targeted chemotherapy. The nanomicelles prepared in this study demonstrated excellent biocompatibility and responsiveness to stimuli. PCL-SS-MPC nanomicelles displayed drug release in response to GSH and pH, resulting in high DOX release at GSH 10 mM and pH 5. Our findings, supported by MTT, flow cytometry, and confocal laser scanning microscopy, demonstrated that AuS-PM-TMTM-DOX micelles effectively induced apoptosis and enhanced cellular uptake in MCF7 and MDA-MB231 cell lines. The cytotoxic effects of AuS-PM-DOX/US on cancer cells were approximately 38 % higher compared to AuS-PM-DOX samples at a concentration of IC50 0.68 nM. This increase in cellular toxicity was primarily attributed to the promotion of apoptosis. The introduction of disulfide linkages in AuSNPs resulted in increased ROS production when exposed to ultrasound stimulation, due to a reduction in GSH levels. Compared to other commercially available nanosensitizers such as titanium dioxide, exposure of AuS-PM to ultrasound radiation (1.0 W/cm, 2 min) significantly enhanced cavitation effects and resulted in 3 to 5 times higher ROS production. Furthermore, laboratory experiments using human breast cancer cells (MDA-MB-231, MCF7) demonstrated that the toxicity of AuS-PM in response to ultrasound waves is dose-dependent. The findings of this study suggest that this formulated nanocarrier holds great potential as a viable treatment option for breast cancer. It can induce apoptosis in cancer cells, reduce tumor size, and display notable therapeutic efficacy.
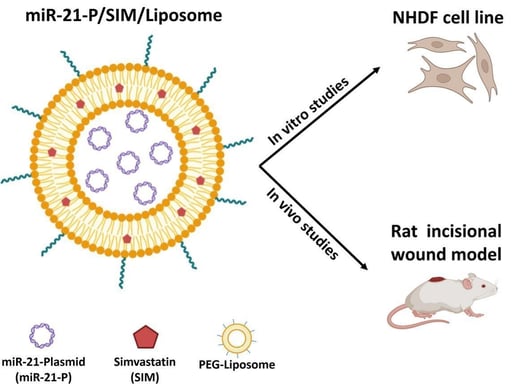
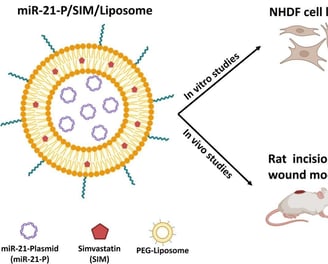
Co-delivery of simvastatin and microRNA-21 through liposome could accelerates the wound healing process
The gene delivery approach, mainly microRNAs (miRNA) as key wound healing mediators, has recently received extensive attention. MicroRNA-21 (miR-21) has strongly impacted wound healing by affecting the inflammation and proliferation phases. Previous studies have also demonstrated the beneficial effect of simvastatin on wound healing. Therefore, we designed a dual-drug/gene delivery system using PEGylated liposomes that could simultaneously attain the co-encapsulation and co-delivery of miRNA and simvastatin (SIM) to explore the combined effect of this dual-drug delivery system on wound healing. The PEG-liposomes for simvastatin and miR-21 plasmid (miR-21-P/SIM/Liposomes) were prepared using the thin-film hydration method. The liposomes showed 85 % entrapment efficiency for SIM in the lipid bilayer and high physical entrapment of miR-21-P in the inner cavity. In vitro studies demonstrated no cytotoxicity for the carrier on normal human dermal fibroblast cells (NHDF) and 97 % cellular uptake over 2 h incubation. The scratch test revealed excellent cell proliferation and migration after treatment with miR-21-P/SIM/Liposomes. For the in vivo experiments, a full-thickness cutaneous wound model was used. The wound closure on day 8 was higher for Liposomal formulation containing miR-21-P promoting faster re-epithelialization. On day 12, all treated groups showed complete wound closure. However, following histological analysis, the miR-21-P/SIM/Liposomes revealed the best tissue regeneration, similar to normal functional skin, by reduced inflammation and increased re-epithelialization, collagen deposition and angiogenesis. In conclusion, the designed miR-21-P/SIM/Liposomes could significantly accelerate the process of wound healing, which provides a new strategy for the management of chronic wounds.
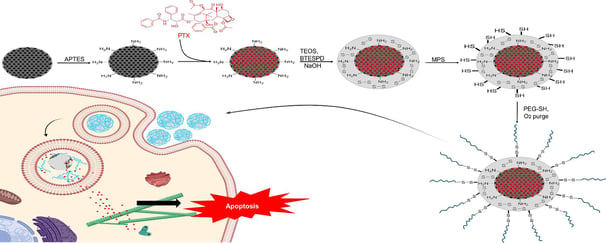
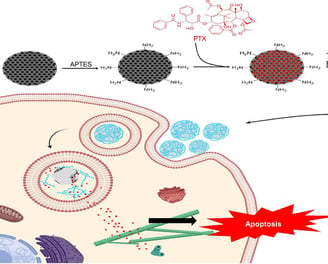
PEGylated mesoporous silica core–shell redox-responsive nanoparticles for delivering paclitaxel to breast cancer cells
Controlling the drug release and restricting its presence in healthy organs is extremely valuable. In this study, mesoporous silica nanoparticles (MSN) as the core, loaded with paclitaxel (PTX), were coated with a non-porous silica shell functionalized with disulfide bonds. The nanoparticles were further coated with polyethylene glycol (PEG) via disulfide linkages. We analyzed the physicochemical properties of nanoparticles, including hydrodynamic size via Dynamic Light Scattering (DLS), zeta potential, X-ray Diffraction (XRD) patterns, Fourier-Transform Infrared (FTIR) spectra, and imaging through Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM). The drug release profile in two distinct glutathione (GSH) concentrations of 2 µM and 10 µM was measured. The cellular uptake of nanoparticles by MCF-7 cell line was determined using Confocal Laser Scanning Microscopy (CLSM) images and flow cytometry. Furthermore, the cell viability and the capability of nanoparticles to induce apoptosis in MCF-7 cell line were studied using the MTT assay and flow cytometry, respectively. Our investigations revealed that the release of PTX from the drug delivery system was redox-responsive. Also, results indicated an elevated level of cellular uptake and efficient induction of apoptosis, underscoring the promising potential of this redox-responsive drug delivery system for breast cancer therapy.